The Scope of the Issue
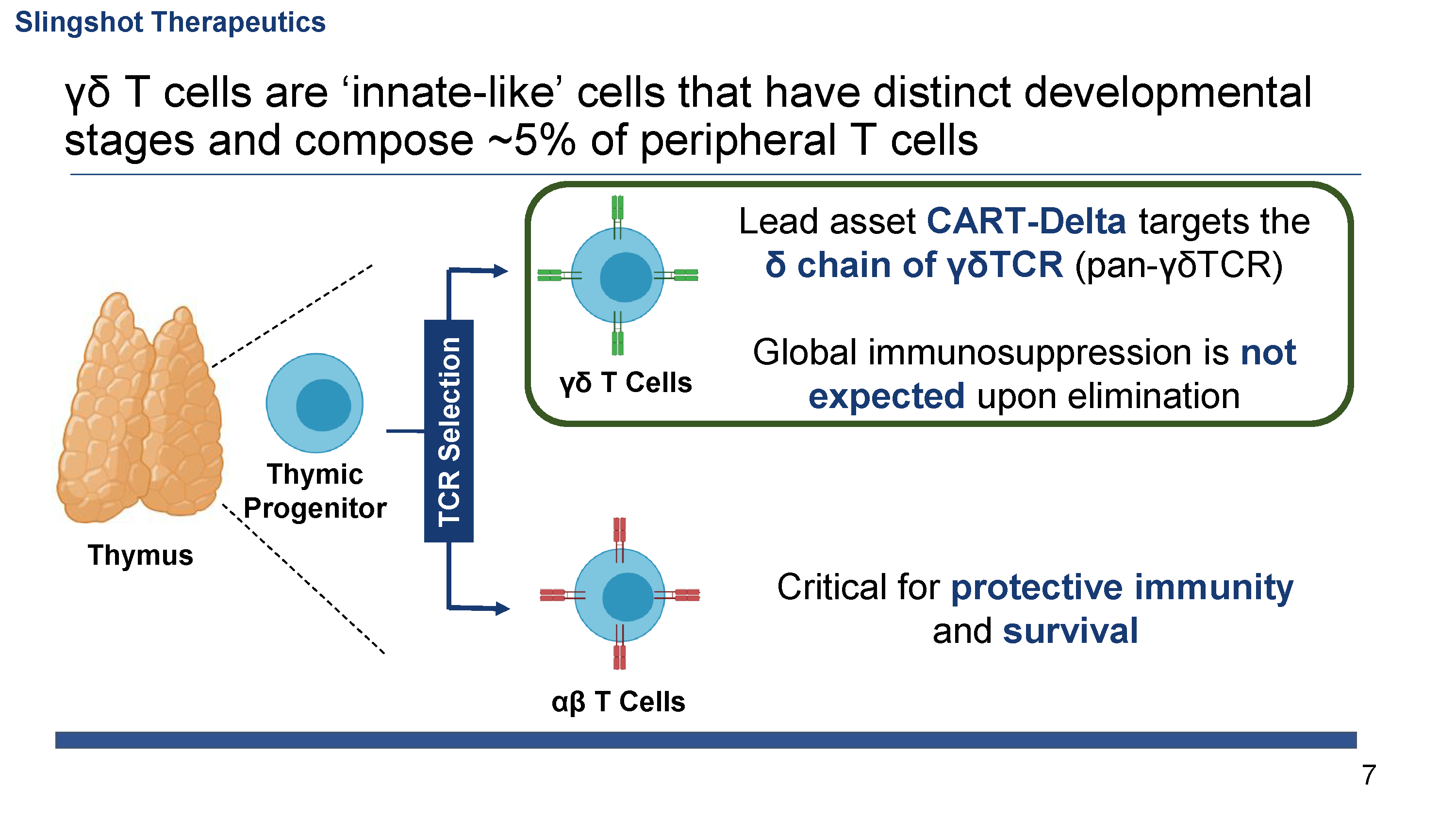
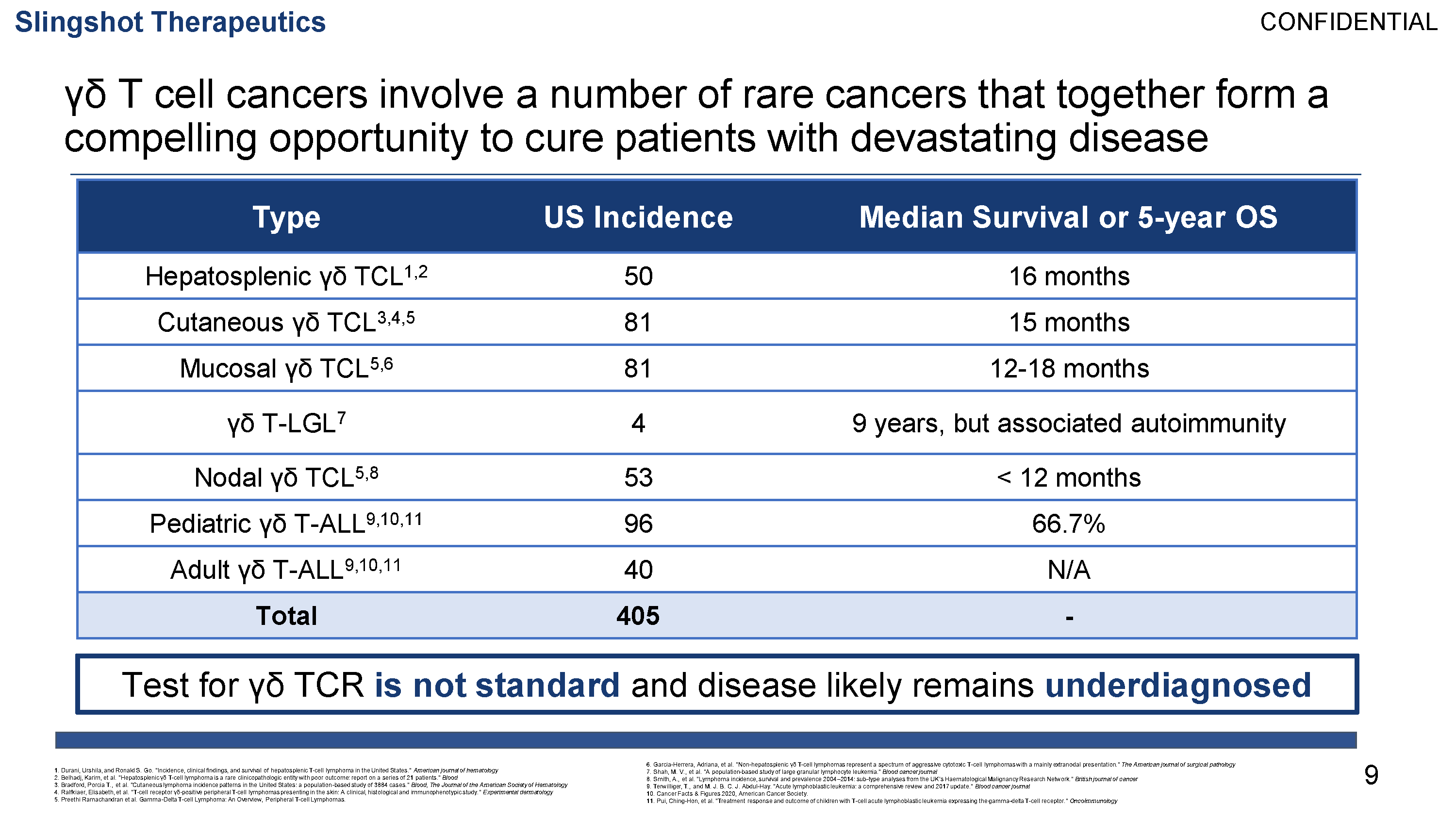
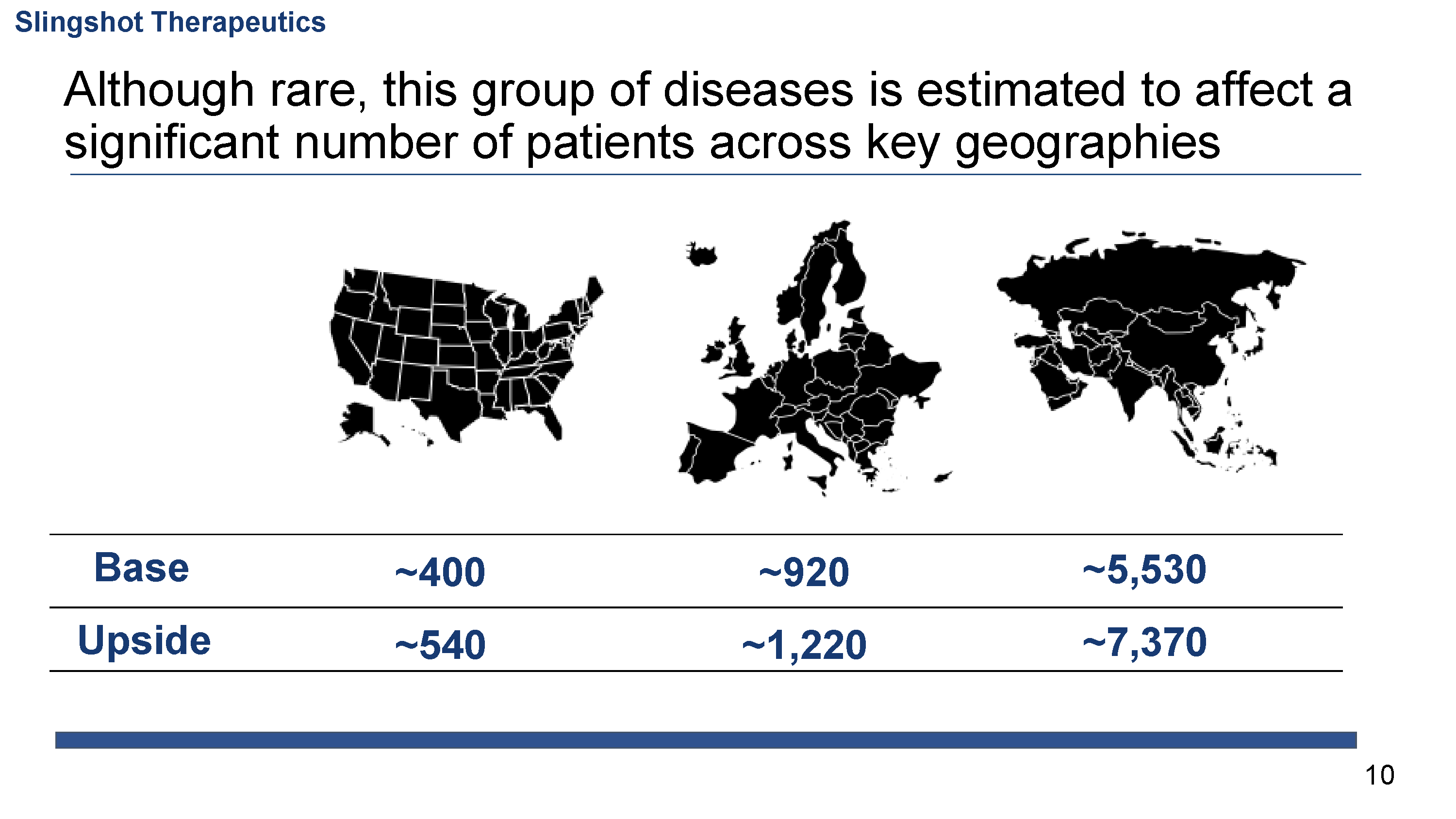
γδ T cell leukemias and lymphomas (γδTCLL) are a group of aggressive neoplasms caused by the clonal proliferation of γδ T cells. γδTCLL is estimated to affect over 9,000 patients per year worldwide. Treatment primarily consists of polychemotherapy and radiation, but due to the highly refractory nature of disease, patients with γδTCLL have a median survival of 15 months, underscoring the extreme unmet need for effective therapy. Additionally, γδ T cells have been implicated in a number of inflammatory and autoimmune diseases including psoriasis, ankylosing spondylitis, and inflammatory myopathies.
Founding Team
Clinical Hypothesis
Key Messages
1
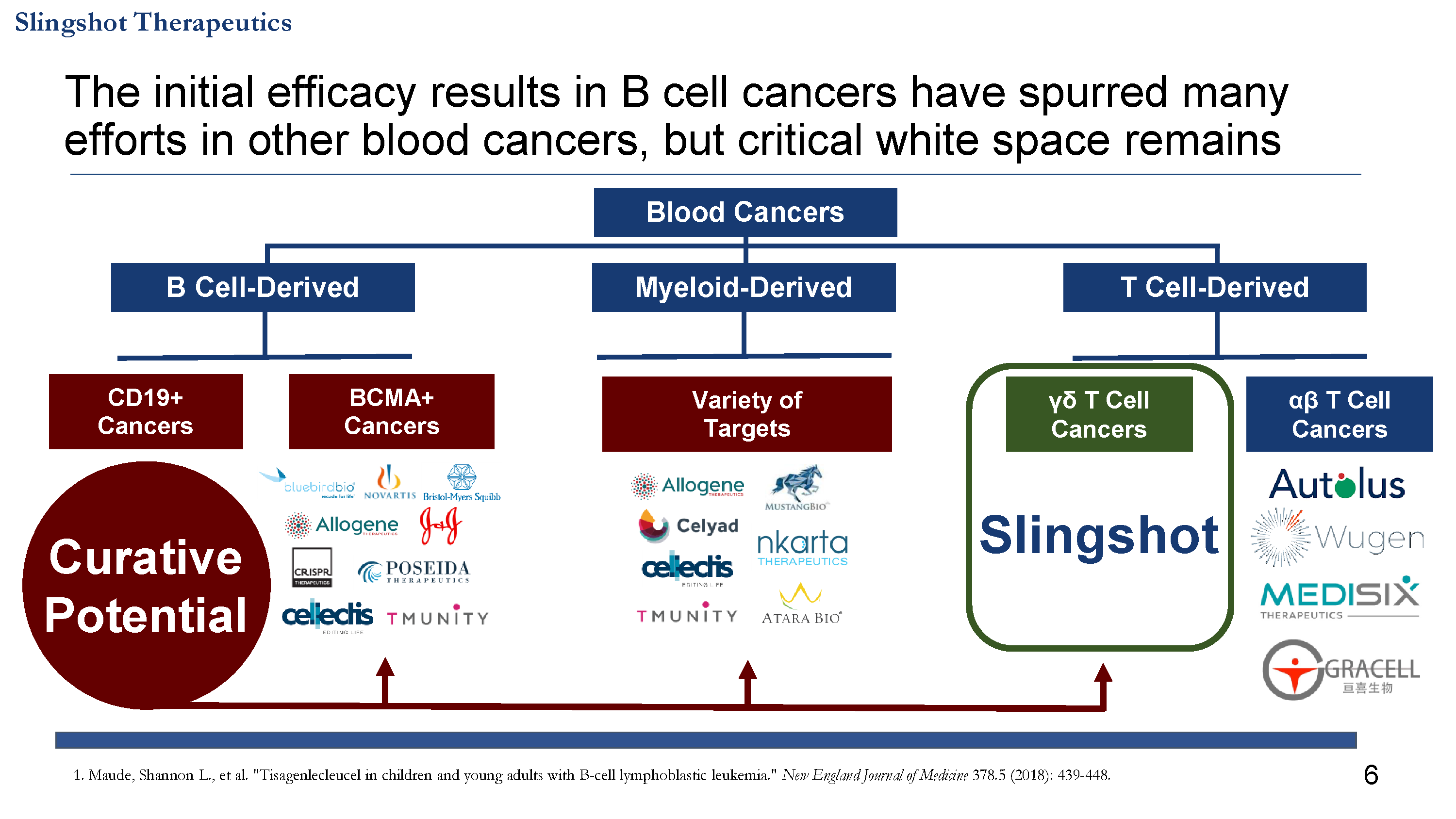
Lead indication focused on curing γδ T cell cancers
- γδ T cell cancers are an ideal target for CAR T cells given past successes in blood cancers, highly restricted expression, and predicted safety profile
- Founding team with experience developing engineered T cell products
2
Demonstrated efficacy and preliminary safety of lead asset in vivo
- Patent filed and licensing negotiations are ongoing with Penn
- Next 12-month value inflection point will be final IND-enabling studies focused on manufacturing and safety
3







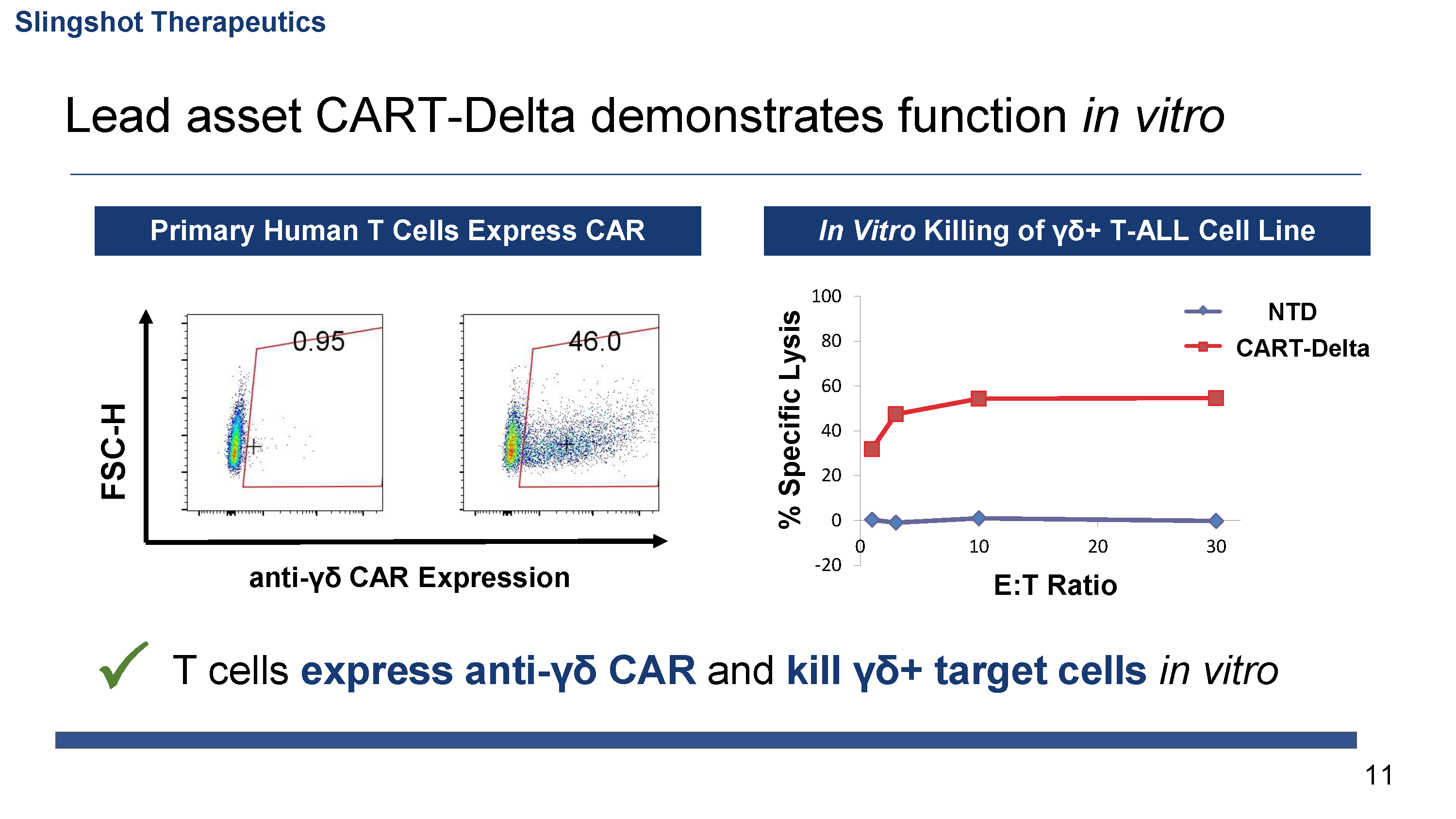
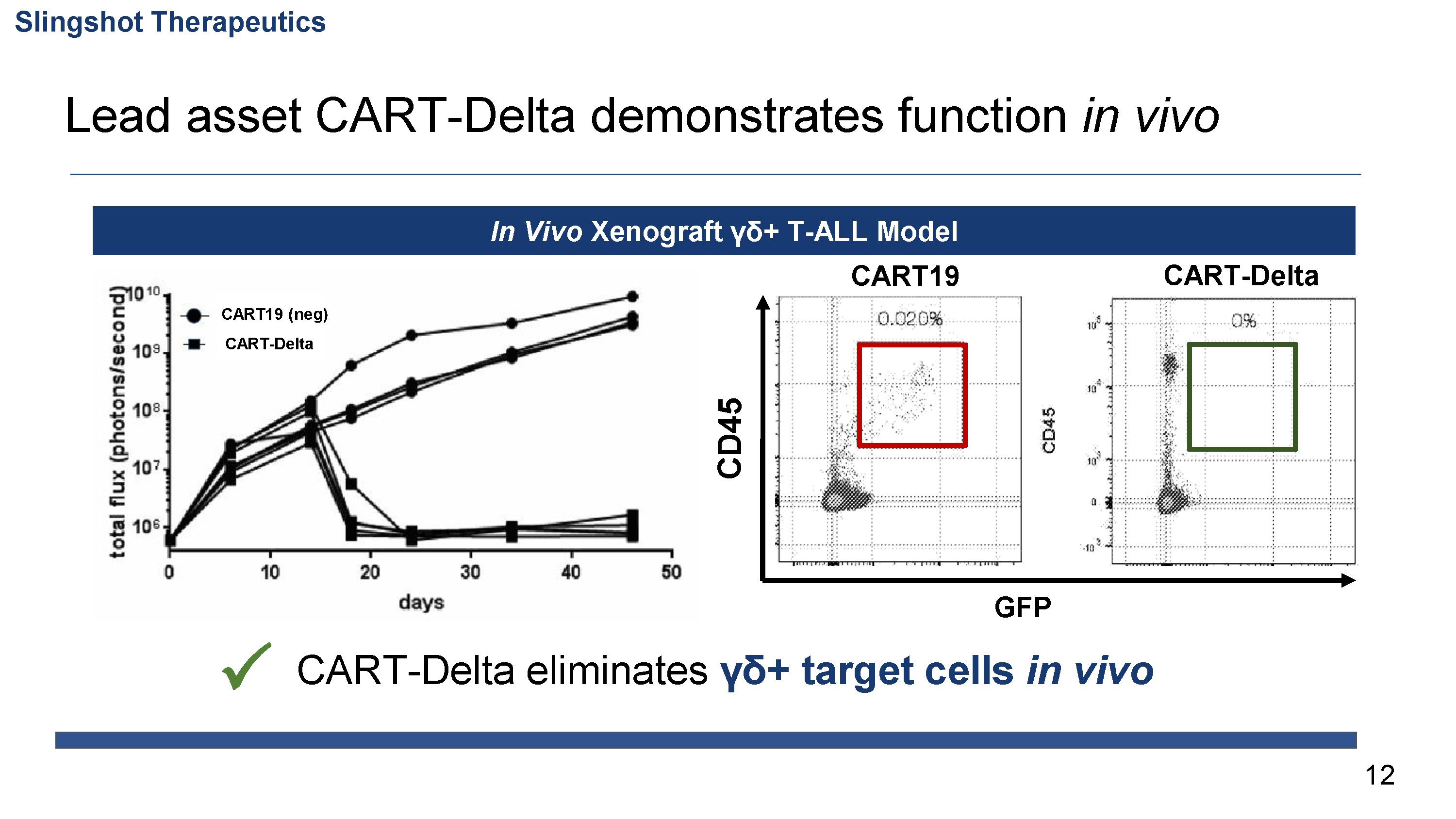
Excellent Work!